PES Capsule Filters
The PoreCap® series of PES capsule filters are ready-to-use sterile grade filtration devices available in 0.1µm and 0.22µm, and different sizes as per requirements. 0.45µm is for bioburden and particulate reduction.
With our advanced PES (Polyethersulfone) Membrane, these filters guarantee optimal bacterial retention and are a go-to choice for filtration of vaccines, injectables, API buffers, water for injections, beverages and lab water purification.
Manufactured in-house in ISO Classified cleanrooms, these devices incorporate highly efficient sterilizing grade Asymmetric Polyethersulfone Membranes safely placed inside a Polypropylene housing.
Crafted with precision & expertise, the PoreCap® PES capsule filters are easy to install & use as they eliminate the need to clean and validate the stainless-steel housing separately.
Membrane Characteristics: Each PoreCap® PES Capsule filter incorporates our high throughput hydrophilic PES membrane that offers excellent chemical compatibility and withstands a wide pH range. Available in single layer or dual layer options, these are absolute retention filters and offer excellent contaminant removal capacity.
APPLICATIONS
- Bioburden reduction
- Sterile filtration for Injectables, biopharmaceutical products, vaccine etc.
- Sterile filtration of APIs, buffer solutions, opthalmic solutions, disinfectants
- Water for injection, purified water and lab water systems
- Filtration of Wine, Beer & other alcoholic beverages
Microbial Validation
Validated as per the ASTM F 838-05 requirements for determining bacterial retention of membrane filters used for liquid filtration
FDA Compliant
Biosafety & Extractables
Integrity Tested
Global Manufacturing Standards
Easy Identification & Tracking
Each filter unit has a unique identification marking which enables quick backtracking
100% Integrity Tested
Guaranteed Specification as per Actual Data
Non Leachable
Ergonomically Designed & Developed
TECHNICAL SPECIFICATIONS
PES Capsule Filters
| Materials of Construction | ||
| Membrane: | Polyethersulfone | |
| Housing: | Polypropylene | |
| Core: | Polypropylene | |
| End Connections: | Polypropylene | |
| Rings: | Silicone (Only with 1/4" MNPT) | |
| Operational Parameters | ||
| Max Temp | 80 °C @ ≤ 2 Kg/cm2 | |
| Max Pressure | 3.5 Kg/cm2 @ 25 °C for forward | |
| 0.7 Kg/cm2 @ 25 °C for reverse | ||
| Sterilizability | 30 autoclave cycles of 30 minutes at 121 °C | |
| Bubble Point: | ||
| 0.10µm: | ≥ 1931 mbar (28 psi) (70% IPA/ water wetted) | |
| 0.22µm: | ≥ 3447 mbar (50 psi) (with water wetted) | |
| 0.45µm: | ≥ 2206 mbar (32 psi) (with water wetted) | |
| Max Air Diffusion Flow (for 10″ Cartridge): | ||
| 0.10µm: | ≤ 30 mL/min @ 3447 mbar (50 psi) | |
| 0.22µm: | ≤ 30 mL/min @ 2482 mbar (36 psi) | |
| 0.45µm: | ≤ 35 mL/min @ 1655 mbar (24 psi) | |
| Microbial Retention: | ||
| 0.22µm: | LRV > 7 for Brevundimonas Diminuta | |
| 0.45µm: | LRV > 7 for Serratia marcescens |
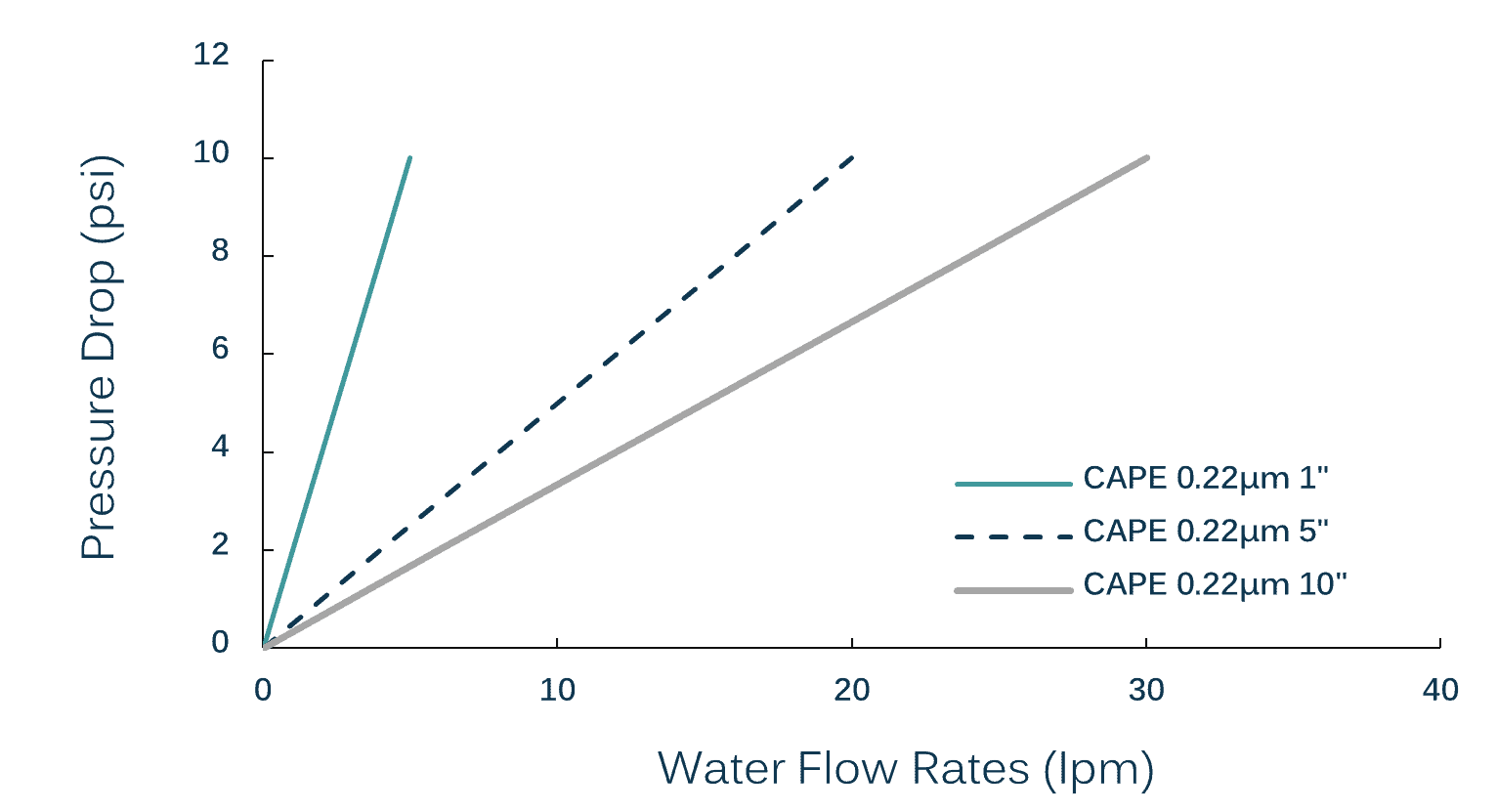
| Effective Filtration Area: | |||||
| Length: | 1″ | 2″ | 5″ | 8″ | 10″ |
| Area: | 0.025m2 | 0.05m2 | 0.10m2 | 0.20m2 | 0.60m2 |
QUALITY ASSURANCE & VALIDATION
All filters are 100% integrity tested and are developed and manufactured under an ISO 9001:2015 certified Quality Management System and designed to be used in cGMP compliant processes.
Microbial Validation
All our Cartridge and Capsule filters are validated as per the ASTM F 838 requirements for determining bacterial retention of sterilizing grade filters
Certificate of Quality
All our products are delivered along with a Certificate of Quality (CoQ) mentioning the materials of construction and testing & quality parameters
Throughput Studies
We help you select the perfect mix of prefilters and final filters by performing small scale filtration and determining the filtration area to reach your desired throughput
Extractables & Leachables
Our Extractables & Leachables testing study is a highly effective way to quantify and assess risks associated with leachable impurities from filters
Why Choose Us
With our extensive knowledge in membrane filtration and a wide range of products available at every stage of the process, along with our exceptional customer service, we are able to establish ourselves as the preferred partner.

Extensive Product Portfolio
At Nupore, we offer a wide range of filtration for your process requirements. Use our quick guide to land instantly at the product of your choice
Proven Membrane Technology

Fast Shipping
Talk To Us
Need assistance choosing the right product for your application, or have a question about ordering from us? Connect with an Expert