Polypropylene Capsule Filters
The PoreCap® series of Polypropylene (PP) capsule filters are specially designed and developed for high particle retention, dirt holding and pre-filtration applications.
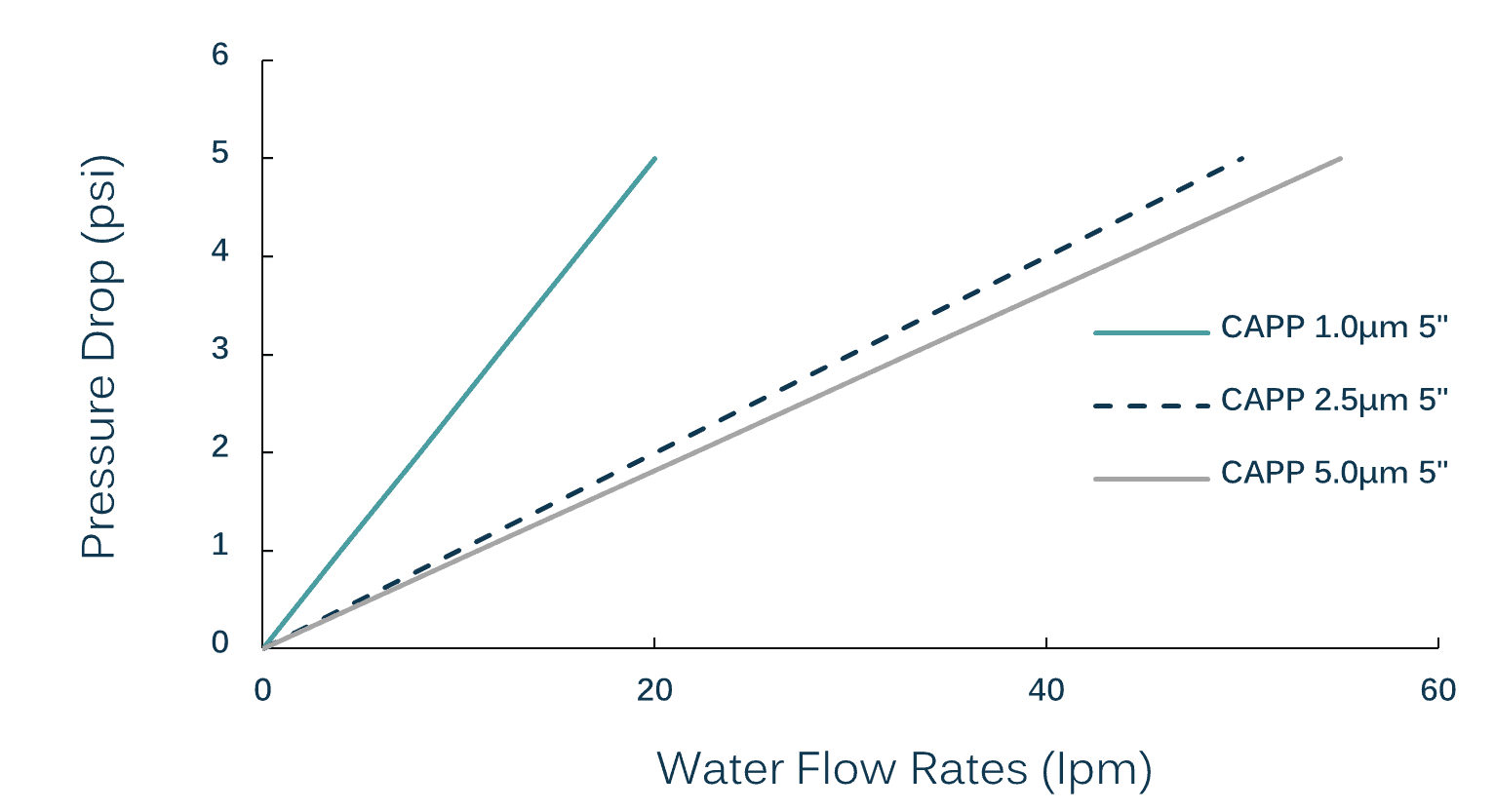
They offer both surface and depth filtration with excellent throughput. High flow Polypropylene capsule filters can be used as pre-filters, final filters or point-of-use filters.
These are tested for retention efficiency, integrityand flow rate, and validated for heat stability, beta ratio, fibre particle release and biosafety.
Membrane Characteristics: Our Polypropylene Capsule Filters incorporate high performance single layer or multilayer Polypropylene membranes which are best suited for turbidity reduction resulting in optimal protection of final sterile grade filters.
Materials of Construction
| Membrane: | Polypropylene |
| Housing: | Polypropylene |
| Core: | Polypropylene |
| End Connections: | Polypropylene |
| Rings: | Silicone (Only with 1/4" MNPT) |
Operational Parameters
| Max Temp: | 80 °C @ ≤ 2 Kg/cm² |
| Max Pressure: | 3.5 Kg/cm² @ 25 °C for forward, 0.7 Kg/cm² @ 25 °C for reverse |
| Sterilizability: | 30 autoclave cycles @125°C |
Sterilization
| ETO: | Yes |
| GAMMA : | Yes |
| Non-sterile: | Yes |
| Non-sterile (Gamma strerilizable): | Yes |
Regulatory Compliance
| Endotoxin releasing: | Contains <0.25 EU/mL as determined by the LAL test |
| Particle release: | Meets the requirements of WFI set by USP |
| Indirect food additive: | Compliant with FDA Indirect Food Additive requirements cited in 21 CFR 177.1520 |
| Material toxicity: | Components meet the requirements of the USP Reactivity Test for Class VI Plastics |
| Quality: | Manufactured in accordance with ISO 9001 and ISO 13485 certified quality management system |
| Extractables: | Tested according to BioPhorum Operations Group (BPOG) guideline. Report is available upon request. |
Drug Specific Filter Validation Services
Through, nSure Validation Services, we offer a complete range of filter validation services to qualify your molecules with our Filters in accordance with the requirements listed in PDA TR#26 and in compliance with international regulatory bodies such as USFDA and EU GMP.








