PTFE Cartridge Filters
PTFE Cartridge filters are sterile grade filtration devices designed to meet the stringent demands of pharmaceutical and food & beverage industries, as these filters guarantee sterile filtration for air and gas applications.
Assembled with our sterilizing-grade PTFE (polytetrafluoroethylene) membranes, the PTFE Cartridge filters are the most recommended filters for Tank Venting and Air Filtration applications as they ensure optimized pressure in the tank while ensuring sterility in the tank.
Manufactured in-house inn ISO Class 8 cleanrooms and designed with a unique construction structure & an advanced pleating technology, these PTFE Cartridge filters are 100% integrity tested and ensure optimized sterilization of air/gases.
Membrane Characteristics: The NuCart® PTFE Cartridge filters incorporate hydrophobic PTFE membrane that offers high flow rates, a high number of sterilization cycles, and excellent chemical resistance in a variety of applications.
Materials of Construction
| Membrane: | Polytetrafluoroethylene |
| Housing: | Polypropylene |
| Core: | Polypropylene |
| End Connections: | Polypropylene |
| Rings: | Silicone (Only with 1/4" MNPT) |
Operational Parameters
| Max Temp: | 80 °C @ ≤ 2 Kg/cm² |
| Max Pressure: | 3.5 Kg/cm² @ 25 °C for forward, 0.7 Kg/cm² @ 25 °C for reverse |
| Autoclavable: | 100 autoclave cycles @125°C, 90 autoclave cycles @135°C |
Bubble Point
| 0.22µm: | ≥ 1380 mbar (20 psi) (with 70% IPA) |
| 0.45µm: | ≥ 690 mbar (10 psi) (with 70% IPA) |
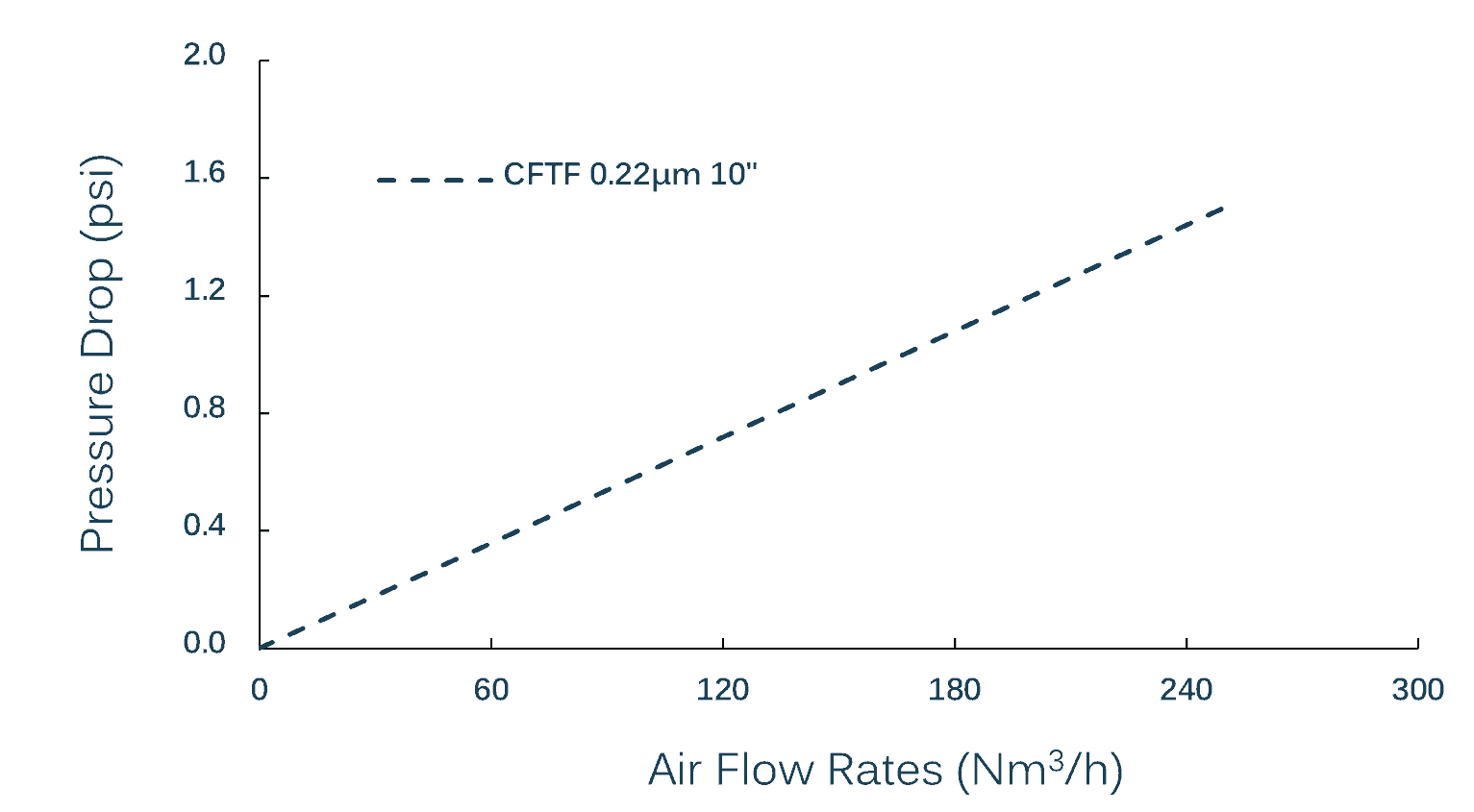
Max Air Diffusion Flow (for 10″ Cartridge)
| 0.22µm: | ≤ 40 mL/min @ 1104 mbar (16 psi) (with 70% IPA) |
| 0.45µm: | ≤ 40 mL/min @ 552 mbar (8 psi) (with 70% IPA) |
Microbial Retention
| 0.22µm: | LRV > 7 for Brevundimonas Diminuta |
| 0.45µm: | LRV > 7 for Serratia marcescens |
Regulatory Compliance
| Endotoxin releasing: | <0.25 EU/mL as determined by the LAL test |
| Particle release: | Meets the requirements of WFI set by USP |
| Non-fiber releasing: | Meets the criteria for a non-fiber releasing filter as per USP |
| Material toxicity: | All components meet the requirements of the USP Reactivity Test for Class VI Plastics Filters meet the requirements of USP Biological Reactivity Tests in Vitro Cytotoxicity |
| Bacterial challenge test: | Filters with the claim passed the bacterial challenge testing using Brevundimonas diminuta (ATCC 19146) at a minimum challenge concentration 1x10⁷ CFU/cm² (0.22 µm) per ASTM F838-20. |
| Extractables: | Tested according to BioPhorum Operations Group (BPOG) guideline. Report is available upon request. |
| Indirect food additive: | Meets the FDA Indirect Food Additive requirements cited in 21 CFR 177.1520 |
| Food contact material: | (EU) No. 1935/2004, (EU) No. 10/2011 |
| Quality: | Manufactured in accordance with ISO 9001 and ISO 13485 certified quality management system |
Drug Specific Filter Validation Services
Through, nSure Validation Services, we offer a complete range of filter validation services to qualify your molecules with our Filters in accordance with the requirements listed in PDA TR#26 and in compliance with international regulatory bodies such as USFDA and EU GMP.







